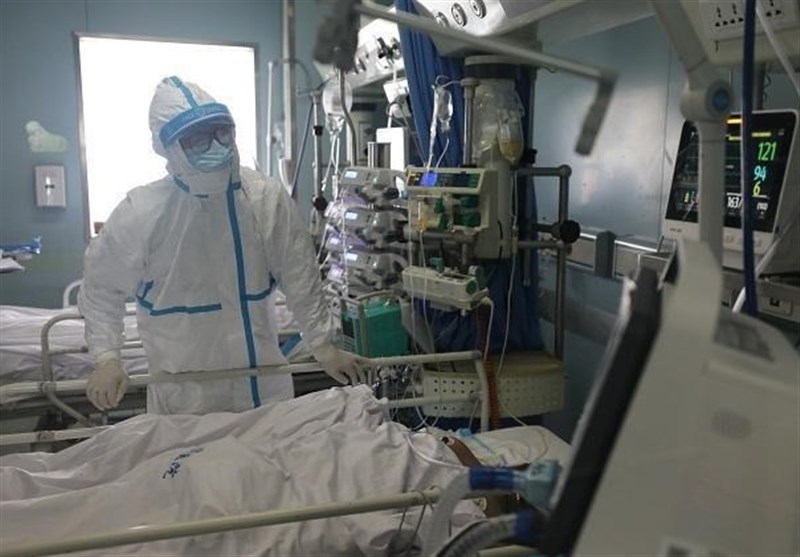
Some Local Beijing Communities Start Giving Elderly COVID-19 Shots
TEHRAN (Tasnim) – Some local communities in Beijing, China, have started giving COVID-19 vaccine doses to people older than 60, even as the city’s disease-control authorities publicly advises this age group against inoculation.
In some countries, including the United States and Britain, people older than 65 are among top priority groups in their COVID-19 vaccination rollouts, as the elderly have a higher risk of death and hospitalization after contracting the virus compared to younger adults.
China’s vaccination program, under which 40.5 million doses had been administered as of Feb. 9, excludes those ages above 59 and those younger than 18, with Chinese vaccine makers citing less complete clinical trial data for minors and the elderly.
According to notices from staffers of a few communities in Beijing’s central Dongcheng district, seen by Reuters, residents older than 60 can go to designated sites to get their shots, without disclosing which the four China-developed vaccines would be available. The vaccinations are not mandatory.
However, an article published online by the Beijing Center for Disease Prevention and Control on Sunday categorized these older than 60 as unsuitable for vaccination, in line with national guidelines.
It is unclear why the Dongcheng district was telling the elderly to be vaccinated.
The city is not fighting the spread of any COVID-19 cluster. Beijing last reported a local case on Jan. 29.
China is due to kick off its annual meeting of parliament on Friday, when thousands of delegates from across the country will gather in Beijing.
The information office of Beijing Municipal People’s Government didn’t immediately respond to a faxed request for comment.
China has four locally developed vaccines approved for general public vaccination, including two from China National Pharmaceutical Group (Sinopharm), one from Sinovac Biotech and one from CanSino Biologics Inc (CanSinoBIO), all of which have been used in smaller-scale vaccination programmes before clearance for wider use.
The formal approvals for vaccines from CanSinoBIO and Sinovac were for people older than 18, without age cap, according to company statements.